Cancer Xenograft Models in Hematologic Research
Xenograft models are an essential component of modern cancer research. By implanting human tumor cells into immunodeficient mice, researchers can replicate the progression and biology of cancer in a living system. These in vivo models make it possible to study tumor growth, evaluate the efficacy of experimental treatments, and observe molecular changes in real-time under conditions that closely mirror human disease.
Cancer xenografts offer a bridge between in vitro discovery and clinical application. In the context of blood-related cancers, they allow for precise assessment of how therapeutic agents behave within the immune system, circulatory system, and bone marrow microenvironment. This is especially important for blood cancers, where disease spread is systemic rather than localized, and therapeutic targeting must be highly specific.
Understanding Blood Cancers
Blood cancers, also known as hematologic malignancies, originate in the blood-forming tissues such as bone marrow and lymph nodes. Unlike solid tumors, these cancers typically involve cells that circulate throughout the body, including white blood cells, plasma cells, and lymphocytes.
Leukemia is a type of blood cancer that arises in the bone marrow and leads to the overproduction of abnormal white blood cells. It is classified into acute and chronic forms, and may originate from either myeloid or lymphoid lineages. Examples include acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), and T‑cell or B‑cell leukemias.
Lymphoma is another category of blood cancer, originating in lymphatic tissues. It involves the transformation and uncontrolled growth of lymphocytes. Subtypes include Burkitt’s lymphoma, follicular lymphoma, diffuse large B‑cell lymphoma (DLBCL), and mantle cell lymphoma. These malignancies can develop in lymph nodes, spleen, and other tissues of the immune system.
Multiple myeloma is a cancer of plasma cells, which are responsible for producing antibodies. It begins in the bone marrow and leads to bone degradation, immune dysfunction, and kidney damage. Unlike leukemias and lymphomas, myeloma cells often localize in bone lesions, but can still be modeled effectively using xenograft approaches.
Supporting Translational Research and Drug Development
Blood cancer xenograft models are critical for bridging the gap between laboratory research and clinical trials. They allow for the preclinical testing of monoclonal antibodies, kinase inhibitors, CAR-T cell therapies, and small molecules targeting the genetic and immunologic pathways of hematologic cancers.
Using xenograft models, researchers can explore not only tumor volume reduction but also deeper mechanistic insights into resistance, relapse, tumor microenvironment interaction, and immune modulation. These studies are essential for understanding how a therapy performs beyond a petri dish and under real biological pressure.
Altogen Labs offers full-service in vivo study execution, from experimental design to endpoint tissue analysis. Their xenograft models help clients generate reliable, reproducible data that supports regulatory submissions, publications, and therapeutic advancement.
HL-60 Xenograft Model
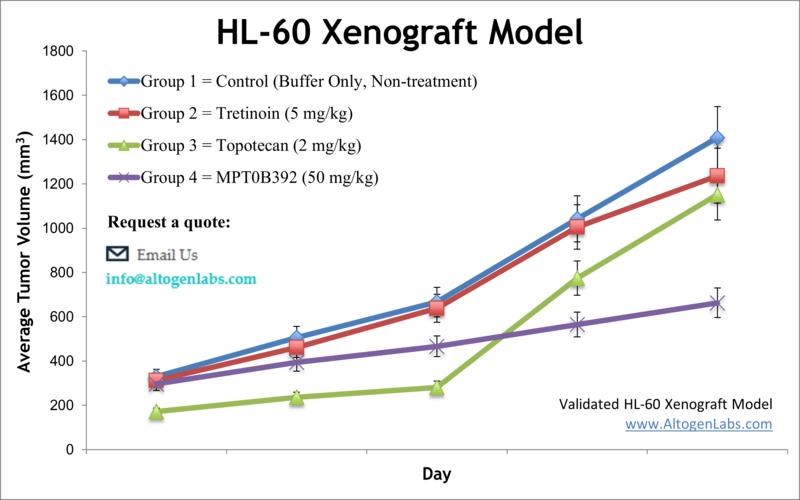
Overview of the HL‑60 Xenograft Model
The HL‑60 xenograft model is a well-established preclinical platform for studying acute myeloid leukemia (AML) in vivo. HL‑60 cells are a human promyelocytic leukemia cell line originally derived from a 36-year-old female patient with AML. These cells are widely used in cancer biology because of their ability to undergo myeloid differentiation and their responsiveness to various pro-apoptotic and chemotherapeutic compounds. The model mimics key features of human leukemia and is widely accepted for drug efficacy testing, differentiation therapy studies, and translational oncology research.
Scientific Background: HL‑60 Cell Line
HL‑60 cells are suspension cells capable of differentiating into granulocytes, monocytes, or macrophage-like cells in response to specific chemical stimuli such as DMSO or retinoic acid. They express surface markers associated with early myeloid progenitors, including CD33 and CD13, and produce measurable responses to compounds targeting DNA synthesis, apoptosis pathways, and inflammatory signals. Their consistent behavior in vitro and tumorigenic potential in vivo make them a valuable tool for modeling hematologic malignancies.
Xenograft Study Protocol
Altogen Labs uses NOD/SCID or athymic BALB/C mice, typically aged 10–12 weeks, to establish subcutaneous HL‑60 xenografts. Cells are prepared at >98% viability and injected (1×10⁶ cells in 100–150 μL Matrigel) into the hind flank. Tumors generally become palpable within 7–10 days, and growth is monitored using digital calipers. Once tumors reach a volume of 50–150 mm³, mice are randomized into dosing groups. Compounds are administered via custom-defined routes and schedules, with tumor size and body weight recorded multiple times per week.
Study duration typically ranges from 3–6 weeks, depending on tumor growth kinetics and therapeutic effects. Upon reaching endpoint criteria (e.g., 2,000 mm³ tumor volume or defined timepoint), mice are ethically euthanized and undergo necropsy.
Endpoint Analysis and Data Output
Every study includes a complete reporting package that documents all phases of the experiment, from baseline tumor growth to final endpoint analysis. Standard deliverables include tumor growth curves, body weight tracking, gross pathology findings, and photographic records of tumor and tissue samples. Altogen Labs offers a suite of downstream services that includes histopathology, immunohistochemistry, RNA and protein extraction, gene expression profiling, and Western blot analysis. Optional customization allows researchers to define specific molecular or phenotypic readouts aligned with their project goals. All procedures are performed in GLP-compliant facilities under IACUC oversight to ensure high standards of scientific rigor, data reproducibility, and animal care.
Applications and Use Cases
The HL‑60 xenograft model supports a wide range of applications in leukemia and hematologic malignancy research. It is ideal for evaluating drug efficacy in vivo, investigating mechanisms of drug resistance, and assessing compounds that induce differentiation or apoptosis in leukemia cells. This model is also used in pharmacokinetics and pharmacodynamics studies, toxicity screening, and biomarker discovery. Its reproducible tumor growth and well-documented lineage biology provide a robust platform for testing both small molecule drugs and biologics.
Request an Instant Quote: https://altogenlabs.com/request-quote/hl60-xenograft-model-services/
Learn more: HL-60 Xenograft Model
K562 Xenograft Model
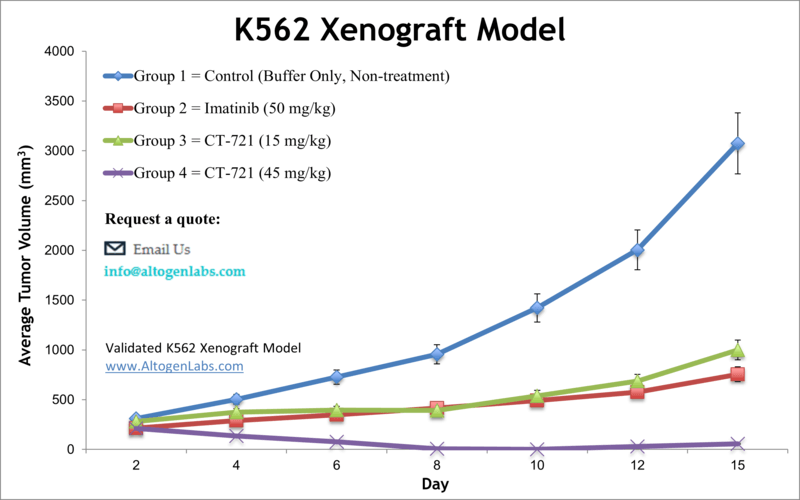
Overview of the K562 Xenograft Model
The K562 xenograft represents a reliable and well-characterized in vivo platform for evaluating chronic myelogenous leukemia (CML) and acute myeloid leukemia (AML) therapies. K562 cells, originally derived from a 53‑year‑old CML patient in blast crisis, are human erythroleukemic and highly undifferentiated, making them a versatile model that can mimic various myeloid phenotypes in vivo. Subcutaneous implantation of these cells into immunodeficient mice creates reproducible solid tumors that are easily measured and suitable for therapeutic efficacy studies .
Scientific Background: K562 Cell Biology
K562 cells share key characteristics with both granulocytes and erythrocytes, carrying the Philadelphia chromosome and expressing the BCR‑ABL1 oncogene. They lack MHC class I expression, rendering them particularly sensitive to natural killer (NK) cell activity, making them a standard target for NK cytotoxicity assays. Their ease of culture, low clumping behavior, and ability to differentiate in response to chemical stimuli such as imatinib or phorbol esters enhance their adaptability across experimental platforms
Xenograft Study Design
Altogen Labs establishes the K562 xenograft model in NOD/SCID or athymic BALB/C mice aged 10 to 12 weeks. Cells are prepared in log-phase at greater than 98 percent viability and mixed with Matrigel prior to subcutaneous injection (1×10^6 cells in 100 µL). Tumor formation is typically detected within 7 to 10 days, with daily volume measurements commenced once tumors reach approximately 75–150 mm³. Animals are randomized into treatment groups and dosed according to the client’s protocol. Tumor volumes are recorded daily with calipers, while body weights are monitored three times per week. Study endpoints are reached when pre-defined criteria—such as a 2,000 mm³ tumor volume or ethical considerations—are met. Animals are then euthanized following GLP and IACUC-compliant protocols.
Endpoint Analysis and Deliverables
Each study includes comprehensive documentation including tumor volume data, animal weight records, and photographic evidence. Necropsies are performed, with tumors weighed, imaged, and processed as required. Altogen offers expanded services such as histopathology, immunohistochemistry, RNA/protein extraction, and molecular analyses. Tissue samples can be snap-frozen, fixed for histology, or processed for nucleic acid isolation based on client specifications.
In Vivo Phenotype and Disease Relevance
K562 xenograft tumors are typically vascularized solid masses with tumor cell morphology reflective of human CML/AML and can demonstrate metastasis to bone marrow and circulation in some cases. In selectin-competent mouse models, K562 infiltration of bone marrow and blood is evident; selectin-deficient models show reduced propagation and prolonged survival, underscoring the model’s utility in studying leukemic cell homing and metastasis.
Request an Instant Quote: https://altogenlabs.com/request-quote/k562-xenograft-model-services/
Learn more: K-562 Xenograft Model
MOLM-13 Xenograft Model
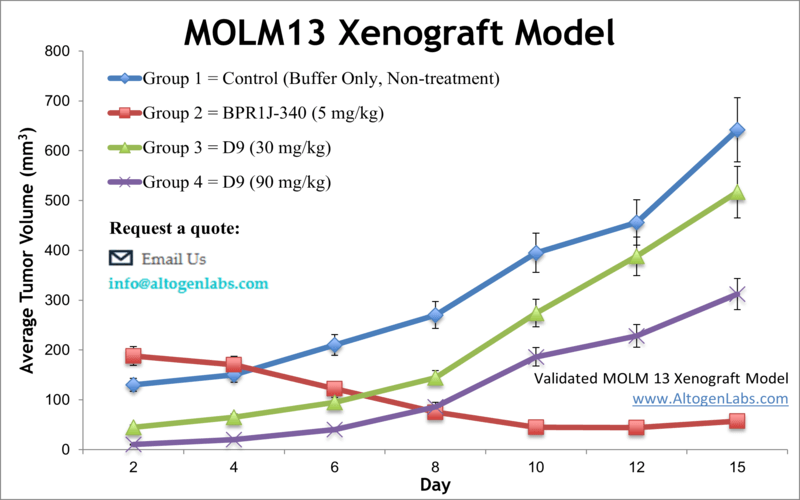
Model Overview
The MOLM‑13 xenograft model employs a human acute myeloid leukemia (AML) cell line derived from a relapsed AML patient. Known for carrying the MLL‑AF9 fusion and FLT3‑ITD mutation, MOLM‑13 cells are an aggressive and clinically relevant choice for evaluating AML-targeted therapies. These cells grow rapidly in immunocompromised rodents, forming solid tumors ideal for in vivo efficacy studies and modeling drug resistance.
Scientific Background
Established from peripheral blood of a 20‑year‑old AML-M5a patient, MOLM‑13 cells harbor the MLL‑AF9 translocation and trisomy 8, among other chromosomal anomalies. The FLT3-ITD mutation, present in many aggressive AML forms, makes this cell line a standard for assessing FLT3-targeting compounds in preclinical research. MOLM‑13-derived xenografts recapitulate signaling pathways and responses associated with aggressive leukemia, including sensitivity to kinase inhibitors and susceptibility to differentiation therapies.
Study Design by Altogen Labs
Altogen Labs uses 10–12 week old NOD/SCID or athymic BALB/C mice for establishing MOLM‑13 xenografts. Cells are prepared to ≥99% viability and mixed with Matrigel for subcutaneous injection (typically 1×10⁶ cells in 100–200 µL). Tumors form rapidly, reaching a volume of approximately 80–120 mm³ before animals are randomized into treatment arms. Tumor volumes are measured daily with digital calipers while body weights are tracked three times per week. Treatment regimens, dosing routes, and schedules are customized per client specifications. Animals are euthanized according to ethical criteria when tumors reach pre-established endpoints.
Endpoint Measurements and Data Reporting
At study conclusion, Altogen Labs offers a complete report inclusive of tumor growth curves, body weight records, and statistical analyses. Tumors are collected, weighed, imaged, and processed—either frozen or fixed depending on downstream assays. Optional analyses include histopathology, immunohistochemistry, RNA/protein isolation, gene expression profiling, and metabolic or blood chemistry assays. All work occurs in GLP-compliant settings under IACUC supervision, ensuring compliance with regulatory and ethical standards.
Research Applications and Relevance
This model supports evaluation of AML therapies including FLT3 inhibitors, differentiation agents, and immunotherapy candidates. It captures key aspects of aggressive leukemia, such as rapid tumor growth and genetic fidelity to patient-derived disease. MOLM‑13 xenografts are used in studies of tumor growth inhibition (TGI), tumor growth delay (TGD), pharmacokinetics/pharmacodynamics (PK/PD), toxicity screening, and evaluation of resistance mechanisms. Their aggressive growth profile enables fast turnarounds and meaningful treatment comparisons.
Request an Instant Quote: https://altogenlabs.com/request-quote/molm13-xenograft-model-services/
Learn more: MOLM-13 Xenograft Model
MOLT-4 Xenograft Model
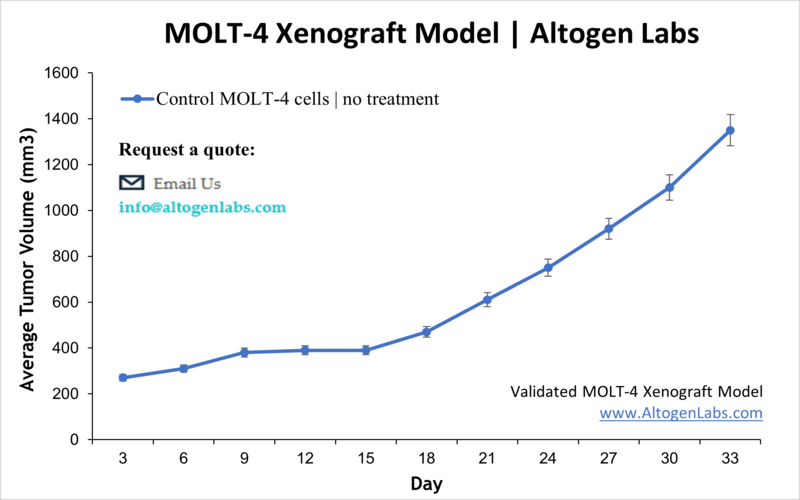
Overview of the MOLT‑4 Xenograft Model
The MOLT‑4 xenograft model provides a dynamic and clinically relevant in vivo platform for studying human T‑cell acute lymphoblastic leukemia (T‑ALL). Originating from a 19‑year‑old patient in relapse, the MOLT‑4 cell line reliably engrafts in immunodeficient mice to form palpable tumors that respond to therapeutic interventions. This model is particularly useful for evaluating novel anti‑leukemia agents and understanding T‑ALL treatment resistance and relapse mechanisms.
Scientific Background: MOLT‑4 Cell Line
MOLT‑4 cells are characterized as hypertetraploid human T‑lymphoblasts derived from relapsed T‑ALL and carry a G→A mutation in p53 codon 248. These cells express high levels of T‑cell markers such as CD1, CD2, CD3, CD4, CD5, CD6, and CD7, as well as terminal deoxynucleotidyl transferase (TdT). Their aggressive phenotype and expression profile make them a robust model for studying immunophenotypic responses, signal transduction, and chemotherapy resistance.
Study Protocol by Altogen Labs
Studies are conducted using immunodeficient mouse strains (e.g., NOD/SCID or athymic nude), with 10–12‑week‑old animals used for consistent engraftment. MOLT‑4 cells are cultured in log phase, ensuring ≥98% viability before suspension in Matrigel and subcutaneous implantation (1×10^6 cells in 100 µL). Palpable tumors typically form within 7–10 days. Once tumors reach 120–160 mm³, animals are randomized, and therapeutic compounds are administered on a client-defined schedule. Tumor volumes are measured daily, and body weights are recorded three times weekly. Studies proceed until tumors reach ethical endpoints, at which point animals are humanely euthanized .
Endpoint Analysis and Data Delivery
Altogen Labs provides a detailed final report that includes tumor growth curves, body weight data, statistical analyses, necropsy observations, and high-resolution imaging of tumor samples. Tumors and associated tissues are harvested and processed for downstream analyses including histopathology, immunohistochemistry, RNA/protein extraction, and molecular assays. Samples may be preserved via RNAlater, snap freezing, or formalin fixation based on client requirements. All workflows are conducted in GLP-compliant facilities under IACUC oversight.
Applications and Disease Relevance
The MOLT‑4 xenograft model is widely used to test differentiation therapies, apoptosis inducers, chemotherapeutics targeting T‑ALL, and novel biologics. Its high expression of T‑cell markers enables mechanistic studies of T‑cell receptor signaling and immune escape. The hypertetraploid genotype and relapsed origin support investigations into resistance mechanisms and relapse biology. This model is also an established platform for tumor growth inhibition (TGI) and delay (TGD) analysis, PK/PD assessments, and combinatorial drug testing.
Request an Instant Quote: https://altogenlabs.com/request-quote/
Learn more: MOLT4 Xenograft Model
A20 Xenograft Model
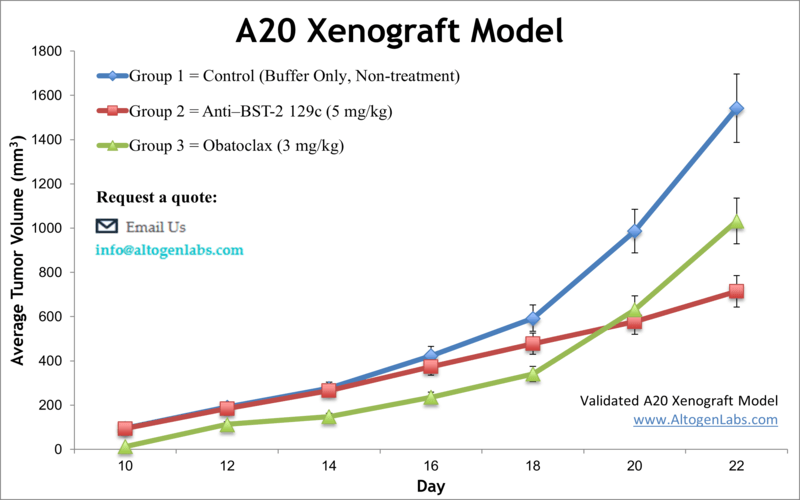
Overview of the A20 Xenograft Model
The A20 xenograft model employs a murine B‑cell lymphoma cell line derived from spontaneous reticulum cell sarcoma in aged BALB/c mice. This syngeneic model retains a fully competent host immune system, enabling in-depth studies on immuno-oncology interventions that engage both tumor and immune components. Tumors form predictably and respond robustly to immunomodulatory agents including checkpoint inhibitors and radiation therapy, making this model a valuable asset for evaluating combination strategies and novel immunotherapies.
Biological Background of A20 Cells
A20 cells originated from a spontaneous B‑cell lymphoma in a BALB/c mouse and are widely used to model non-Hodgkin B‑cell malignancies. These cells express high levels of PD‑L1 and respond effectively to immune-targeting treatments such as anti‑PD‑1, anti‑PD‑L1, and anti‑GITR antibodies. They exhibit moderate doubling times of approximately 4–5 days in vivo, allowing for sufficient therapeutic windows to assess tumor growth inhibition, immune infiltration, and survival outcomes.
Study Design by Altogen Labs
Altogen Labs performs A20 xenograft studies in immunocompetent BALB/c mice. Tumors are established through subcutaneous inoculation of A20 cells, and dosing with test compounds begins once tumors are palpable or reach a defined volume. During the study, tumor dimensions and animal body weights are recorded regularly. Clinical signs and tumor progression are monitored daily. At endpoint, typically determined by tumor size or health criteria, mice undergo necropsy. Tumor tissues are collected for photographic documentation, weighing, and further molecular analysis.
Endpoint Measurements & Data Reporting
Upon study completion, Altogen Labs delivers comprehensive datasets that include tumor growth curves, body weight records, necropsy findings, and image documentation. Optional downstream analyses include histopathology, immunohistochemistry, isolation of RNA/protein, gene expression analysis, and immune profiling. All study activities are carried out in GLP-compliant facilities under strict IACUC oversight, ensuring regulatory compliance and data integrity.
Immuno-Oncology Insights & Application
Because A20 tumors grow in an immunocompetent environment, this model is particularly valuable for evaluating immune checkpoint blockade, costimulatory agonists, CAR-T constructs, cancer vaccines, and combination therapies involving radiation or conventional chemotherapies. It supports assessments of anti-tumor responses, T-cell/macrophage infiltration, and immunomodulatory mechanism interrogation. Dual-mode versions of the model—involving orthotopic or systemic (i.v.) administration and luciferase-labeled cells—enable tracking of metastasis, tumor burden, and long-term treatment outcomes.
Request an Instant Quote: https://altogenlabs.com/request-quote/a20-xenograft-model-services/
Learn more: A20 Xenograft Model
Daudi Xenograft Model
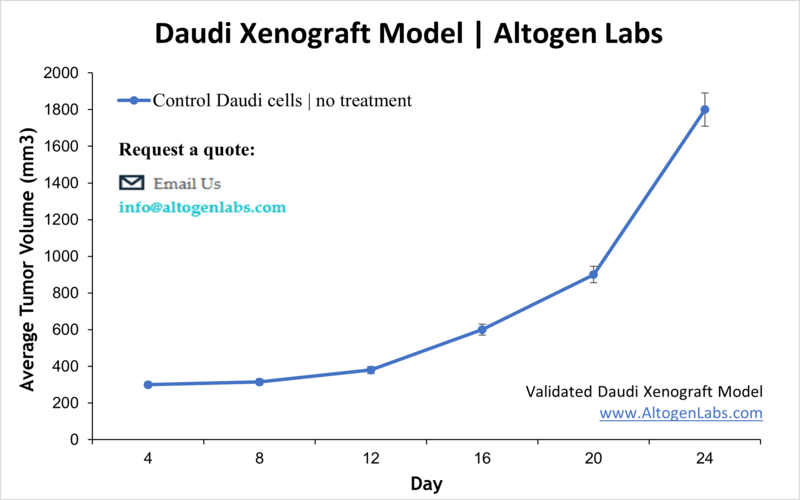
Overview of the Daudi Xenograft Model
The Daudi xenograft model is a well-established in vivo platform for studying Burkitt’s lymphoma and other high-grade B-cell lymphomas. Derived from a human patient with Burkitt’s lymphoma, Daudi cells are lymphoblast-like and represent one of the earliest human B-cell lines successfully adapted to tissue culture. When injected into immunodeficient mice, these cells reliably form subcutaneous tumors, offering a translational model for evaluating novel therapeutic agents, especially monoclonal antibodies, small-molecule inhibitors, and immune-targeted therapies.
Scientific Background: Daudi Cell Line
Daudi cells are Epstein-Barr virus (EBV)-positive and possess several unique biological features that make them ideal for preclinical studies. As mature B lymphocytes, they express surface immunoglobulin, MHC class I and II molecules, and complement receptors. Their consistent expression of CD19 and CD20 surface markers makes them particularly suitable for testing anti-CD20 therapeutics such as rituximab. Additionally, their EBV-positive status allows researchers to investigate virus-host interactions and viral oncogenesis in the context of lymphoma progression. Because Daudi cells are derived from Burkitt’s lymphoma—a highly aggressive B-cell malignancy with characteristic MYC translocations—they also serve as a platform to study deregulated cell proliferation, metabolic reprogramming, and apoptotic pathways.
Xenograft Study Protocol at Altogen Labs
Altogen Labs conducts Daudi xenograft studies in NOD/SCID or athymic nude mice, typically aged 10 to 12 weeks. The Daudi cells are maintained under strict quality control conditions and harvested during logarithmic growth to ensure high viability before implantation. Approximately one million viable cells, suspended in a Matrigel matrix, are injected subcutaneously into the hind flank of each mouse. Tumors usually become palpable within 7 to 10 days, and animals are randomized into treatment groups when tumors reach approximately 100 to 150 cubic millimeters. Tumor measurements are taken with digital calipers several times per week, alongside regular monitoring of body weight and overall health. Study duration is customized according to the therapeutic regimen and experimental goals. When ethical or volume-based endpoints are reached, animals are humanely euthanized in accordance with IACUC-approved protocols.
Endpoint Analysis and Reporting
Upon study completion, Altogen Labs provides a comprehensive report that includes tumor growth kinetics, body weight data, high-resolution imaging, necropsy observations, and tumor measurements. Tissue samples are collected, weighed, and preserved according to the client’s intended downstream applications. Available post-study services include histological evaluation, immunohistochemistry, flow cytometry, qRT-PCR, RNA/protein extraction, and ELISA. The study is conducted in GLP-compliant, pathogen-free facilities with full adherence to regulatory standards and ethical oversight.
Research Applications and Relevance
The Daudi xenograft model is widely used to evaluate drug candidates targeting surface antigens, intracellular kinases, and immune checkpoints involved in B-cell malignancies. Its compatibility with monoclonal antibody-based therapeutics makes it ideal for testing anti-CD20 and anti-CD19 agents. Researchers can also use this model to study lymphoma microenvironment dynamics, tumor immune evasion mechanisms, and resistance to frontline therapies. Due to its EBV positivity, the model can support research into virus-associated oncogenesis and latent viral protein expression in a tumor setting.
Request an Instant Quote: https://altogenlabs.com/request-quote/
Learn more: Daudi Xenograft Model
Raji Xenograft Model
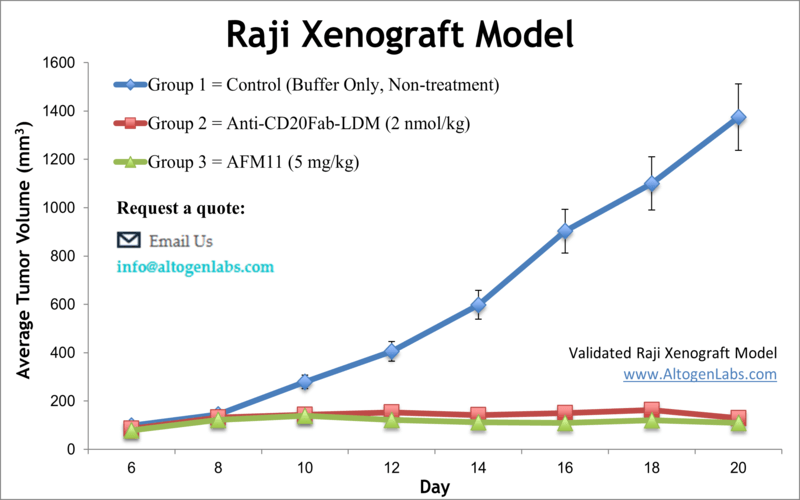
Overview of the Raji Xenograft Model
The Raji xenograft model provides an aggressive, immunodeficient in vivo system for studying human Burkitt’s lymphoma and related B-cell malignancies. Derived from the peripheral blood of an 11-year-old patient with Burkitt’s lymphoma, Raji cells retain characteristics of mature B lymphocytes and have been extensively used in oncology research. When implanted into suitable mouse models, Raji cells generate solid tumors that serve as a robust platform for preclinical drug testing, including antibody-based therapies and targeted small molecules.
Scientific Background: Raji Cell Line
Raji cells are Epstein-Barr virus (EBV)-positive and carry hallmark mutations found in high-grade B-cell lymphomas, such as c-MYC translocations that drive unchecked proliferation. These cells express key B-cell surface antigens including CD19, CD20, CD22, and HLA-DR, making them a valuable model for therapies targeting B-cell surface markers. Raji cells are also used in immunotherapy studies due to their expression of MHC class I and II molecules, as well as their susceptibility to immune-mediated cytotoxicity. Their EBV positivity allows researchers to explore interactions between viral oncogenes and tumor progression mechanisms in vivo.
Xenograft Study Design by Altogen Labs
Altogen Labs establishes Raji xenografts using immunodeficient mice, typically NOD/SCID or athymic nude strains, aged between 10 and 12 weeks. Raji cells are harvested during log-phase growth at ≥98% viability and suspended in a matrix such as Matrigel before subcutaneous injection into the flank of each mouse. Tumors typically begin to form within one week and are closely monitored until reaching a target size for randomization. Tumor volume and body weight are recorded at least three times weekly. Treatment regimens—including compound type, route of administration, and schedule—are fully customized to meet specific experimental objectives. Mice are ethically euthanized once tumors reach study endpoints or if predefined health criteria are met.
Endpoint Analysis and Study Output
Altogen Labs delivers a full data package that includes tumor growth curves, body weight tracking, digital imaging, necropsy reports, and tumor tissue measurements. Clients can request a variety of follow-up analyses such as histology, immunohistochemistry, flow cytometry, protein or RNA extraction, and gene expression profiling. Optional immune cell analysis is available when using humanized or co-engrafted models. All work is conducted in GLP-compliant facilities with IACUC-approved protocols, ensuring consistent, high-quality results suitable for regulatory documentation or publication.
Research Applications and Utility
The Raji xenograft model supports evaluation of a wide spectrum of anti-cancer agents, particularly monoclonal antibodies such as anti-CD20 (e.g., rituximab) and anti-CD19 compounds. Its aggressive growth rate and stable phenotype make it ideal for screening novel therapies and combination regimens. Because the Raji cell line is EBV-positive, the model also enables investigation into virus-associated lymphomagenesis and the effects of therapies targeting latent viral proteins. It is frequently used in studies focused on B-cell receptor signaling, immune escape, apoptosis, and chemotherapy resistance mechanisms.
Request an Instant Quote: https://altogenlabs.com/request-quote/raji-xenograft-model-services/
Learn more: Raji Xenograft Model
EL4 Xenograft Model
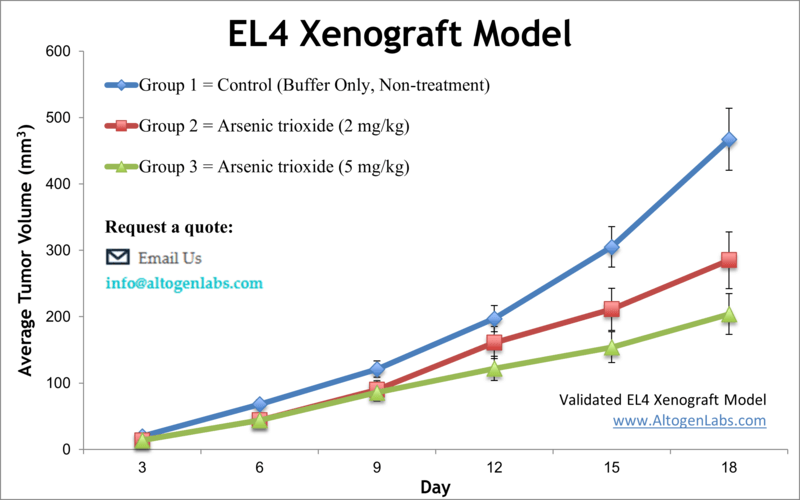
Overview of the EL4 Xenograft Model
The EL4 xenograft model is a widely used syngeneic system for studying murine T‑cell lymphoma in immunocompetent mice. Derived from a chemically induced lymphoma in C57BL/6 mice, EL4 cells enable researchers to evaluate anti-tumor therapies within a fully functional immune system. This model is particularly valuable for preclinical assessment of immunotherapies, checkpoint inhibitors, cytokine treatments, radiation, and combination regimens, providing a translationally relevant platform for studying T‑cell mediated oncogenesis and tumor-immune interactions.
Scientific Background: EL4 Cell Line
EL4 cells are a murine lymphoma cell line that originated from a C57BL/6 mouse and represent an undifferentiated T‑lymphoblastic phenotype. These cells express T‑cell surface markers such as CD3 and CD4 and exhibit high mitotic activity and tumorigenic potential. Because they are syngeneic to the C57BL/6 mouse strain, EL4 cells allow tumor development in a host with an intact immune system. This feature is essential for modeling the dynamics of immune surveillance, T-cell activation, and resistance to immunotherapy. EL4 cells have also been engineered in various experimental systems to express tumor antigens or human immune markers, enhancing their utility in targeted therapy research.
Xenograft Study Protocol at Altogen Labs
Altogen Labs conducts EL4 xenograft studies using young adult C57BL/6 mice, typically 6 to 10 weeks old. EL4 cells are prepared in log-phase growth, achieving high viability before injection. Each mouse receives a subcutaneous flank injection of one million EL4 cells suspended in a Matrigel or PBS matrix. Tumor growth is closely monitored, and once tumors reach a volume of approximately 100 mm³, animals are randomized into treatment cohorts. Test compounds are administered according to client specifications, with common routes including intravenous, intraperitoneal, or subcutaneous delivery. Tumor volumes and animal body weights are recorded multiple times per week, and any signs of distress are documented in compliance with animal welfare protocols. At study endpoint—defined either by tumor size, health status, or duration—mice are humanely euthanized for tissue collection and analysis.
Endpoint Analysis and Data Output
A complete data package is provided to each client, including tumor growth kinetics, body weight tracking, visual tumor documentation, necropsy findings, and tumor weights. Tissues are collected for histological and molecular analysis as needed. Optional services include immunohistochemistry, flow cytometry, cytokine profiling, qRT-PCR, RNA and protein extraction, and T-cell infiltration analysis. The EL4 model supports a range of immune phenotyping studies and is well-suited for experiments that assess immune memory, T-cell function, and tumor rejection. All work is conducted in GLP-compliant, pathogen-free facilities under IACUC-approved protocols.
Applications and Research Utility
The EL4 xenograft model is commonly used in immuno-oncology to evaluate the therapeutic effects of immune checkpoint blockade, T-cell agonists, CAR-T constructs, oncolytic viruses, and vaccine-based immunotherapies. Its compatibility with radiation and chemotherapeutic regimens allows for the investigation of synergistic effects between traditional and novel cancer treatments. Because EL4 tumors grow in a fully immune-competent host, researchers can also study mechanisms of immune suppression, T-cell exhaustion, and tumor escape. This model is applicable to both mechanistic investigations and drug efficacy validation in a biologically relevant setting.
Request an Instant Quote: https://altogenlabs.com/request-quote/el4-xenograft-model-services/
Learn more: EL4 Xenograft Model
DOHH2 Xenograft Model
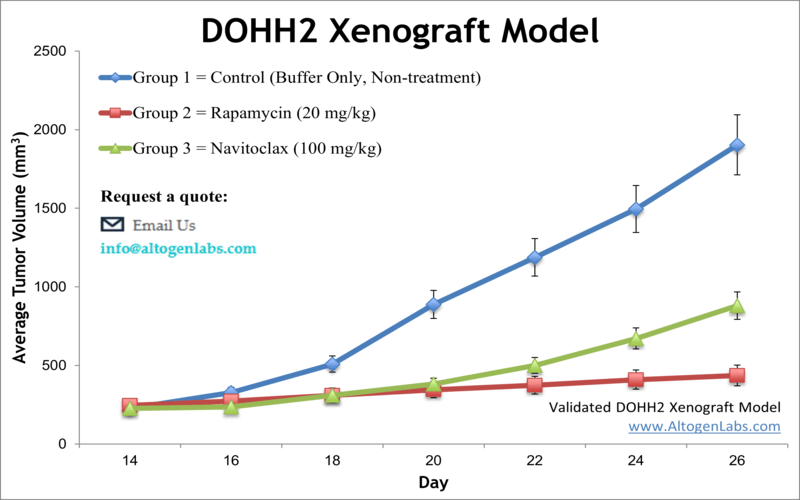
Overview of the DOHH2 Xenograft Model
The DOHH2 xenograft model is an established in vivo system used to study human B‑cell non-Hodgkin lymphoma. Derived from a diffuse large B-cell lymphoma patient, the DOHH2 cell line exhibits aggressive growth characteristics and expresses high levels of B‑cell markers including CD20 and BCL‑2. When implanted into immunodeficient mice, DOHH2 cells form solid tumors suitable for testing targeted therapies, monoclonal antibodies, small-molecule inhibitors, and chemotherapy combinations. The model offers a reproducible and clinically relevant tool for evaluating anti-lymphoma agents in preclinical settings.
Scientific Background: DOHH2 Cell Line
DOHH2 cells are Epstein–Barr virus (EBV)–positive and represent a transformed follicular lymphoma phenotype. These cells harbor genetic alterations associated with therapy resistance and malignant transformation, including BCL‑2 overexpression and MYC activation pathways. Their stable expression of CD19 and CD20 allows for rigorous evaluation of CD20-targeting therapeutics such as rituximab and biosimilar agents. The cell line is also responsive to apoptosis-inducing compounds, making it especially useful in studies focused on BCL‑2 inhibition and caspase signaling. The combination of high proliferative capacity and consistent tumorigenicity supports detailed investigations into lymphoma growth and regression mechanisms.
Xenograft Study Protocol at Altogen Labs
Altogen Labs conducts DOHH2 xenograft studies in immunocompromised mice such as NOD/SCID or athymic nude strains, typically aged 10 to 12 weeks. DOHH2 cells are cultured under optimized conditions and prepared at high viability before injection. Approximately one million cells are suspended in a mixture of PBS and Matrigel and injected subcutaneously into the flank of each mouse. Tumors generally become palpable within one week post-injection, after which animals are randomized into control and treatment groups.
Clients can specify the treatment regimen, including compound type, dose, administration route, and schedule. Tumor volume is measured three times per week using digital calipers, and animal weights are monitored to assess treatment tolerability. Studies typically continue until tumors reach a maximum allowable volume or other experimental endpoints, at which time animals are humanely euthanized and tissues collected for analysis.
Endpoint Analysis and Study Output
At the conclusion of the study, Altogen Labs provides a full report including tumor growth curves, body weight data, images of excised tumors, and necropsy observations. Tumor tissues can be processed and preserved according to the client’s analytical goals—frozen for RNA and protein extraction, fixed for histology and immunohistochemistry, or embedded for detailed microscopic examination. Optional analyses include flow cytometry, qPCR, Western blotting, gene expression profiling, and immune cell infiltration assays. All procedures are carried out in GLP-compliant laboratories under IACUC-approved animal welfare protocols.
Research Applications and Use Cases
The DOHH2 xenograft model is especially suited for testing monoclonal antibodies targeting CD19 and CD20, BCL‑2 inhibitors such as venetoclax, and combination strategies that induce programmed cell death in lymphoma cells. It enables assessment of tumor response, pharmacokinetics, and drug-resistance mechanisms in B-cell malignancies. Researchers frequently use this model for preclinical validation of new therapeutic agents before progressing to clinical trials, making it a critical step in drug development pipelines for lymphomas.
Request an Instant Quote: https://altogenlabs.com/request-quote/dohh2-xenograft-model-services/
Learn more: DOHH2 Xenograft Model
KARPAS 299 Xenograft Model
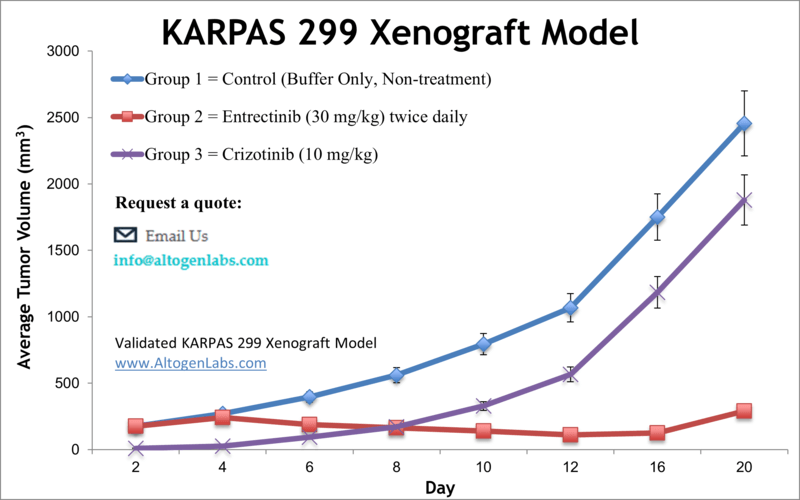
Overview of the KARPAS‑299 Xenograft Model
The KARPAS‑299 xenograft model is a highly specialized preclinical tool for studying anaplastic large cell lymphoma (ALCL), particularly ALK‑positive subtypes. Derived from a human lymphoma patient, KARPAS‑299 cells harbor the t(2;5)(p23;q35) chromosomal translocation, which results in the oncogenic NPM‑ALK fusion protein. This model is widely used to evaluate the efficacy of ALK inhibitors, signal transduction blockers, and combination regimens targeting this aggressive form of peripheral T‑cell lymphoma. When implanted into immunodeficient mice, KARPAS‑299 cells consistently generate tumors, providing a robust and clinically relevant system for therapeutic screening and mechanism-of-action studies.
Scientific Background: KARPAS‑299 Cell Line
KARPAS‑299 cells represent a human ALK‑positive ALCL line originating from a male patient with systemic lymphoma. The hallmark of this cell line is the constitutive expression of the NPM‑ALK fusion protein, which drives malignant transformation through activation of multiple signaling cascades including STAT3, PI3K/AKT, and MAPK. These cells express CD30, a TNF receptor family member and diagnostic marker for ALCL, as well as epithelial membrane antigen (EMA) and cytoplasmic granules typical of large cell lymphoma morphology. The aggressive behavior of the cell line and its defined molecular alterations make KARPAS‑299 ideal for targeted drug development, resistance studies, and biomarker discovery.
Xenograft Study Protocol at Altogen Labs
Altogen Labs conducts KARPAS‑299 xenograft studies in immunodeficient mouse strains such as NOD/SCID or athymic nude mice, typically between 8 and 12 weeks of age. KARPAS‑299 cells are maintained in culture to ensure optimal growth phase and viability before subcutaneous implantation. One million cells, suspended in a small volume of Matrigel or buffered saline, are injected into the flank region of each mouse. Tumor development begins within 7 to 10 days, at which point animals are randomized into treatment and control groups.
Test articles are administered according to the study design, which can include oral, intravenous, or intraperitoneal delivery. Tumor volume is measured three times per week using digital calipers, and body weights are tracked to monitor general health and toxicity. The study continues until tumors reach ethical endpoints or predefined criteria are met, after which animals are euthanized and samples collected for analysis.
Endpoint Analysis and Data Reporting
Altogen Labs provides a full scientific report that includes tumor volume progression, body weight data, necropsy observations, and digital imaging of tumors. Harvested tissues are preserved and processed based on client specifications, including snap freezing, formalin fixation, or embedding for histopathological analysis. Available downstream services include immunohistochemistry, Western blotting, ELISA, RNA and protein extraction, and molecular profiling of ALK signaling pathways. Additional studies such as pharmacokinetics and tumor penetration of therapeutic agents can be integrated into the workflow. All procedures are conducted in GLP-compliant laboratories under IACUC oversight to ensure reproducibility and regulatory alignment.
Research Applications and Use Cases
The KARPAS‑299 xenograft model is particularly well suited for evaluating ALK-targeted therapies such as crizotinib, ceritinib, alectinib, and novel generation ALK inhibitors. It also supports the study of CD30-directed therapeutics, including antibody-drug conjugates and immune-stimulatory agents. Researchers use this model to investigate mechanisms of drug resistance, signal pathway inhibition, apoptosis induction, and combination treatment efficacy. Its defined genetic background and responsiveness to targeted therapies make it a critical model for translational ALCL research and drug development.
Request an Instant Quote: https://altogenlabs.com/request-quote/karpas-299-xenograft-model-services/
Learn more: KARPAS 299 Xenograft Model
Ramos Xenograft Model
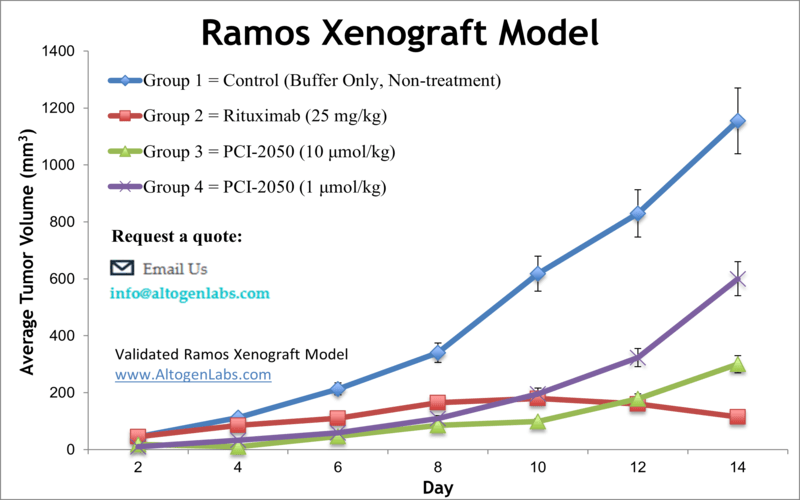
Overview of the Ramos Xenograft Model
The Ramos xenograft model offers a powerful platform for studying human Burkitt’s lymphoma in an in vivo setting. Derived from a 3-year-old male patient with American-type Burkitt’s lymphoma, Ramos cells are characterized by high proliferative activity, stable phenotype, and strong tumorigenicity when implanted into immunodeficient mice. This model is widely used for testing targeted therapies, monoclonal antibodies, apoptosis-inducing agents, and combination regimens relevant to B-cell non-Hodgkin lymphoma.
Scientific Background: Ramos Cell Line
Ramos cells are EBV-negative human B lymphocytes that have been extensively studied due to their unique immunophenotype and genetic stability. Unlike many other Burkitt’s lymphoma cell lines, Ramos cells are derived from a mature B-cell population and exhibit surface immunoglobulin expression, including IgM and light chains. They carry hallmark translocations of Burkitt’s lymphoma involving the c-MYC oncogene, leading to dysregulated cell growth and metabolism. Ramos cells express CD19, CD20, and CD22, making them particularly suitable for evaluating anti-CD20 therapies such as rituximab, as well as BCL-2 family inhibitors and PI3K pathway antagonists.
Xenograft Study Protocol at Altogen Labs
Altogen Labs establishes Ramos xenograft tumors in immunocompromised mouse strains such as NOD/SCID or athymic nude mice, typically 6 to 12 weeks of age. Tumors are initiated by subcutaneous injection of approximately one million viable Ramos cells, suspended in a matrix such as PBS or Matrigel, into the flank region. Tumor formation typically occurs within 7 to 10 days, at which point animals are randomized into treatment groups based on tumor volume.
Clients specify the route and schedule of drug administration, which may include intravenous, intraperitoneal, or oral delivery. Tumor volume and animal weight are measured multiple times per week. Mice are monitored closely throughout the study, and once experimental endpoints are reached—either by tumor size or time point—mice are humanely euthanized for necropsy and tissue collection. All procedures are conducted under GLP conditions and in full compliance with IACUC protocols.
Endpoint Analysis and Deliverables
At study completion, Altogen Labs provides a detailed report including tumor growth curves, group averages, body weight changes, tumor weights, and necropsy documentation. Tumors and other relevant tissues can be preserved for follow-up analysis, such as histology, immunohistochemistry, RNA and protein extraction, flow cytometry, and molecular profiling. The Ramos model supports a broad range of assays related to B-cell signaling, apoptotic response, immune evasion, and therapeutic resistance. Customized endpoint services can include target validation, pharmacodynamic marker analysis, and gene expression quantification.
Applications and Use Cases
The Ramos xenograft model is ideal for evaluating B-cell lymphoma therapies, particularly those targeting CD20, CD22, or the BCR signaling axis. It is commonly used in preclinical testing of small molecules that inhibit PI3K, mTOR, or BTK pathways, as well as monoclonal antibodies and antibody-drug conjugates. The EBV-negative status of the Ramos cell line makes it well-suited for isolating drug effects independent of viral oncogenesis. This model is also valuable for studies of tumor proliferation, apoptosis induction, drug resistance, and immune modulation in the context of Burkitt’s lymphoma.
Request an Instant Quote: https://altogenlabs.com/request-quote/ramos-xenograft-model-services/
Learn more: Ramos Xenograft Model
Granta 519 Xenograft Model
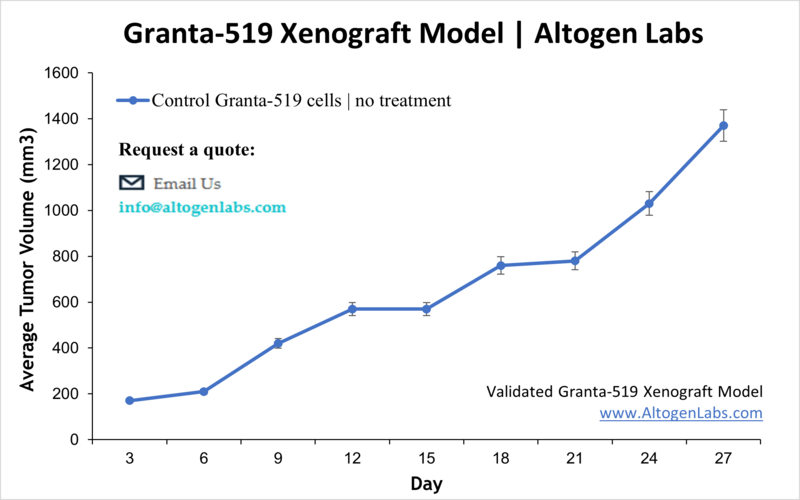
Overview of the Granta‑519 Xenograft Model
The Granta‑519 xenograft model provides a clinically relevant system for studying mantle cell lymphoma (MCL), an aggressive and incurable B-cell malignancy characterized by poor prognosis and frequent relapse. Derived from a human patient with relapsed MCL, Granta‑519 cells harbor hallmark genetic abnormalities, including the t(11;14)(q13;q32) translocation resulting in cyclin D1 overexpression. These cells are known for their aggressive tumor growth and resistance to conventional therapies. When engrafted into immunodeficient mice, Granta‑519 cells form reliable and measurable tumors, making this model suitable for testing targeted therapies, small-molecule inhibitors, and immunotherapeutics.
Scientific Background: Granta‑519 Cell Line
Granta‑519 cells exhibit a mature B-cell phenotype and are characterized by high levels of cyclin D1, a key driver of cell cycle progression in mantle cell lymphoma. This cell line shows activation of NF-κB and PI3K/AKT signaling pathways, contributing to cell survival and resistance to apoptosis. Additionally, Granta‑519 cells express pan-B cell markers including CD19, CD20, CD22, and surface immunoglobulins, making them highly responsive to therapies targeting B-cell receptor (BCR) signaling and anti-CD20 monoclonal antibodies. Due to their well-characterized genetic and phenotypic profile, these cells are a valuable tool for elucidating MCL pathogenesis and evaluating novel agents for clinical translation.
Xenograft Study Protocol at Altogen Labs
Altogen Labs establishes Granta‑519 xenografts in immunodeficient mouse strains such as NOD/SCID or athymic nude mice. Mice are typically 8 to 12 weeks old at the start of the study. Cultured Granta‑519 cells with confirmed viability and phenotype are resuspended in a Matrigel or saline matrix and injected subcutaneously into the flank of each mouse. Tumor formation usually begins within 7 to 10 days, after which animals are randomized into study arms. Clients specify treatment regimens, including compound identity, dose, route of administration, and frequency.
Tumor volumes and animal body weights are measured several times weekly using digital calipers and standard procedures. Treatment continues until tumors reach pre-defined size limits or other experimental endpoints, at which point mice are euthanized humanely and tumors are collected for analysis. All procedures are performed in GLP-compliant laboratories following IACUC-approved protocols to ensure reproducibility, animal welfare, and regulatory alignment.
Endpoint Analysis & Data Reporting
Altogen Labs provides detailed study reports including tumor growth kinetics, body weight trends, necropsy observations, and tumor imaging. Histological and molecular analyses—such as RNA/protein extraction, immunohistochemistry, and flow cytometry—are available. Optionally, pharmacodynamics, immune infiltration profiling, and biomarker evaluation can be incorporated. All work adheres to GLP standards and is overseen by IACUC.
Notable Research Applications
This model is particularly useful for testing small molecules that target Aurora kinases, BTK inhibitors, CD20-directed therapies, and immunomodulatory antibodies. Prior studies have demonstrated efficacy of Aurora A/B inhibitors in combination with chemotherapy, and mTOR inhibition effects monitored via live molecular imaging. The Granta‑519 xenograft also supports evaluation of therapeutic strategies targeting chemokine receptor CCR7 to prevent tumor growth and systemic dissemination.
Request an Instant Quote: https://altogenlabs.com/request-quote/
Learn more: Granta 519 Xenograft Model
RL Xenograft Model
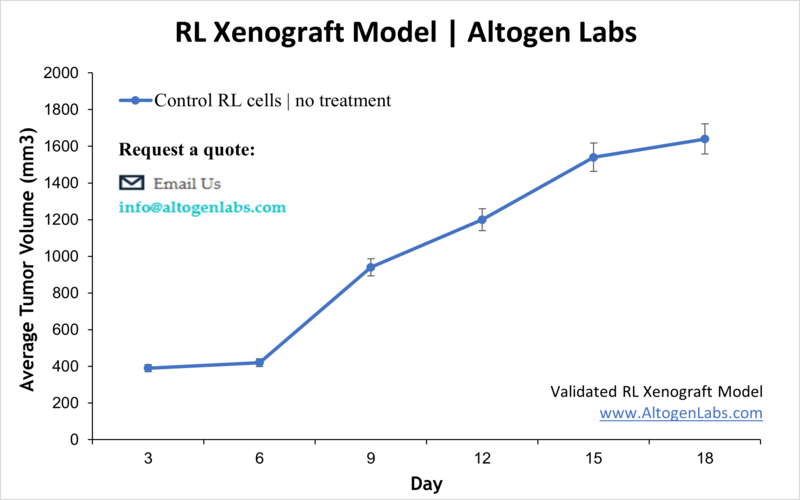
Overview of the RL Xenograft Model
The RL xenograft model is a widely used preclinical tool for studying human follicular B‑cell lymphoma. The RL cell line, derived from a patient’s lymphoma cells, forms reproducible tumors in immunodeficient mice, enabling the evaluation of therapeutic candidates targeting B‑cell malignancies. This model provides a clinically relevant environment to assess efficacy, tumor progression, and drug resistance mechanisms in vivo.
Scientific Background: RL Cell Line
RL cells are derived from a patient with follicular lymphoma and are characterized by expression of B‑cell markers such as CD19 and CD20. They maintain stable growth and phenotype in both in vitro and in vivo settings. The RL cell line is commonly used to study antibody-based therapies, including CD20-targeting agents like rituximab, and novel immunotherapies. Its follicular lymphoma origin makes it an appropriate model for investigating disease biology and response to treatment.
Study Protocol at Altogen Labs
Altogen Labs initiates RL xenografts by injecting approximately two million viable RL cells subcutaneously into immunodeficient mice, often using a Matrigel suspension to support tumor establishment. Tumor growth is monitored regularly, and once tumors reach a defined volume, animals are randomized into treatment groups. The lab administers client-specified therapies through various routes such as intravenous, intraperitoneal, or oral delivery. Tumor size and animal health parameters are tracked throughout the study until ethical endpoints or predefined criteria are met, followed by humane euthanasia and tissue collection.
Endpoint Analysis and Reporting
At study completion, Altogen Labs provides detailed reports featuring tumor growth curves, body weight measurements, and necropsy findings. Tumors and relevant tissues are collected for histology, immunohistochemistry, molecular assays, and biomarker studies according to client needs. Additional analyses such as flow cytometry and gene expression profiling can be included. All processes are conducted in accordance with GLP guidelines and animal welfare standards.
Applications and Research Uses
The RL xenograft model is particularly useful for assessing monoclonal antibodies and antibody-drug conjugates targeting CD20 and other B‑cell markers. It is also employed to evaluate small molecule inhibitors, immune checkpoint modulators, and combination therapies relevant to follicular lymphoma. The model supports studies on tumor biology, therapeutic efficacy, immune interactions, and resistance mechanisms.
Request an Instant Quote: https://altogenlabs.com/request-quote/
Learn more: RL NHL Xenograft Model
OPM2 Xenograft Model
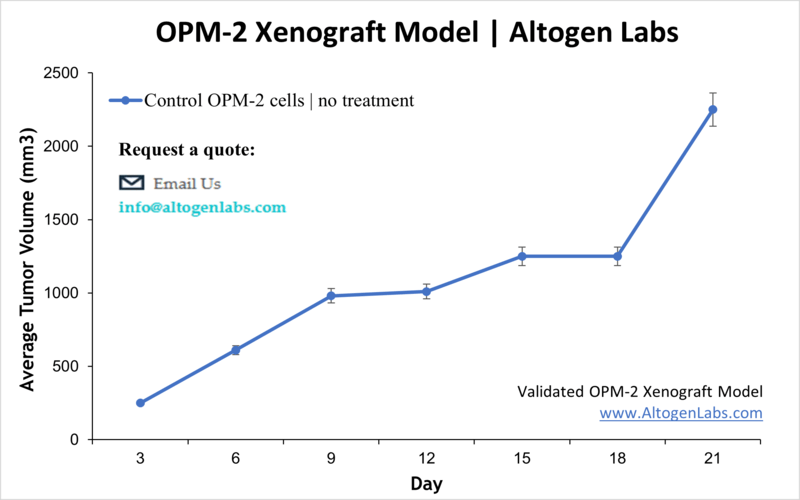
Overview of the OPM2 Xenograft Model
The OPM2 xenograft model is a well-established preclinical platform for investigating multiple myeloma, a malignancy of plasma cells. Derived from a patient with advanced myeloma, OPM2 cells reliably form tumors when implanted into immunodeficient mice. This model provides a valuable system for testing novel therapies, including targeted agents, chemotherapeutics, and immunomodulators, within a biologically relevant in vivo environment. Researchers use it to assess tumor growth, drug efficacy, and mechanisms of resistance in multiple myeloma.
Scientific Background: OPM2 Cell Line
OPM2 is a human multiple myeloma cell line characterized by high proliferation and secretion of immunoglobulins, reflecting the clinical phenotype of plasma cell malignancy. These cells exhibit chromosomal abnormalities typical of myeloma, including translocations involving the immunoglobulin heavy chain locus. OPM2 cells express surface markers such as CD138 and CD38, which are commonly targeted in therapeutic interventions. Their robust growth in vitro and in vivo, as well as consistent tumor formation in xenograft models, make OPM2 a reliable system for preclinical evaluation of multiple myeloma treatments.
Study Protocol at Altogen Labs
At Altogen Labs, OPM2 xenograft studies begin with preparation of viable cells in log-phase growth. Approximately one to two million cells are injected subcutaneously into immunodeficient mice, often suspended in Matrigel to promote tumor establishment. Tumor development typically occurs within 10 to 14 days post-injection. Animals are then randomized into treatment groups, and therapeutic agents are administered according to customized dosing schedules, which may include intravenous, intraperitoneal, or oral routes. Tumor size and animal health parameters are monitored regularly throughout the study.
Endpoint Analysis and Reporting
Upon reaching study endpoints—determined by tumor size or treatment duration—mice are humanely euthanized, and tumors are harvested for comprehensive analysis. Altogen Labs provides detailed reports including tumor growth curves, body weight data, and necropsy findings. Tissue samples can be processed for histological evaluation, immunohistochemistry, molecular profiling, and biomarker analysis according to client needs. Optional assays such as flow cytometry, gene expression, and protein quantification are also available. All procedures adhere to GLP standards and animal welfare regulations.
Applications and Research Utility
The OPM2 xenograft model is ideal for testing novel therapeutics targeting plasma cell malignancies, including proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies against CD38 or CD138, and combination regimens. It allows researchers to study tumor progression, drug response, resistance mechanisms, and tumor microenvironment interactions. This model is also useful for evaluating pharmacodynamics and biomarker expression in multiple myeloma.
Request an Instant Quote: https://altogenlabs.com/request-quote/
Learn more: OPM2 Xenograft Model
H929 Xenograft Model
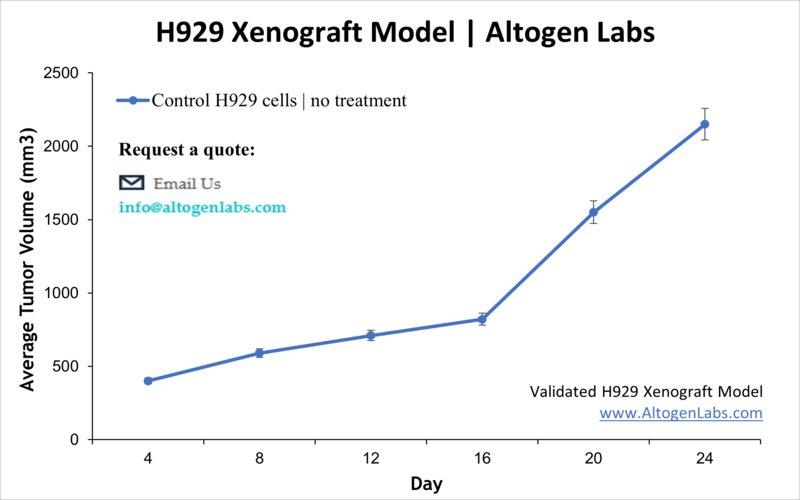
Overview of the H929 Xenograft Model
The H929 xenograft model is an established in vivo system for studying multiple myeloma, a cancer of plasma cells characterized by clonal proliferation within the bone marrow. Derived from a human myeloma patient, H929 cells form reproducible tumors when implanted into immunodeficient mice. This model provides a clinically relevant environment for evaluating the efficacy of new therapeutics, including targeted agents, immunotherapies, and chemotherapeutics, helping researchers to better understand tumor biology and treatment response.
Scientific Background: H929 Cell Line
H929 cells originate from a patient with multiple myeloma and exhibit many characteristics of malignant plasma cells, including high levels of immunoglobulin secretion and expression of surface markers such as CD138 and CD38. The cell line possesses chromosomal abnormalities frequently found in myeloma patients, making it a faithful representation of the disease. H929 cells grow efficiently both in vitro and in vivo, demonstrating aggressive tumor formation in xenograft models, which allows for the detailed study of drug responses and resistance mechanisms.
Study Protocol at Altogen Labs
Altogen Labs establishes H929 xenografts by subcutaneously injecting viable cells, typically one to two million per mouse, suspended in a supportive matrix like Matrigel to promote tumor take and growth. Immunodeficient mice such as NOD/SCID or athymic nude strains are used, with tumor formation generally occurring within 10 to 14 days post-injection. Once tumors reach measurable size, animals are randomized into groups and receive client-specified treatments via appropriate routes (intravenous, intraperitoneal, or oral). Tumor volume and animal health parameters are monitored regularly until study endpoints are reached.
Endpoint Analysis and Reporting
At the conclusion of the study, Altogen Labs provides comprehensive reports including tumor growth curves, body weight tracking, and necropsy findings. Tumor tissues can be processed for histopathology, immunohistochemistry, molecular analyses, and biomarker assessments depending on client requirements. Additional services such as gene expression profiling, protein quantification, and flow cytometry are available. All work is performed under GLP compliance and adheres to animal welfare regulations.
Applications and Research Uses
The H929 xenograft model is ideal for preclinical evaluation of multiple myeloma therapies including proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies targeting CD38 or CD138, and combination treatment regimens. This model facilitates the study of tumor growth inhibition, drug resistance, and mechanisms of action, as well as the assessment of tumor microenvironment interactions relevant to plasma cell malignancies.
Request an Instant Quote: https://altogenlabs.com/request-quote/
Learn more: H929 Xenograft Model